- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
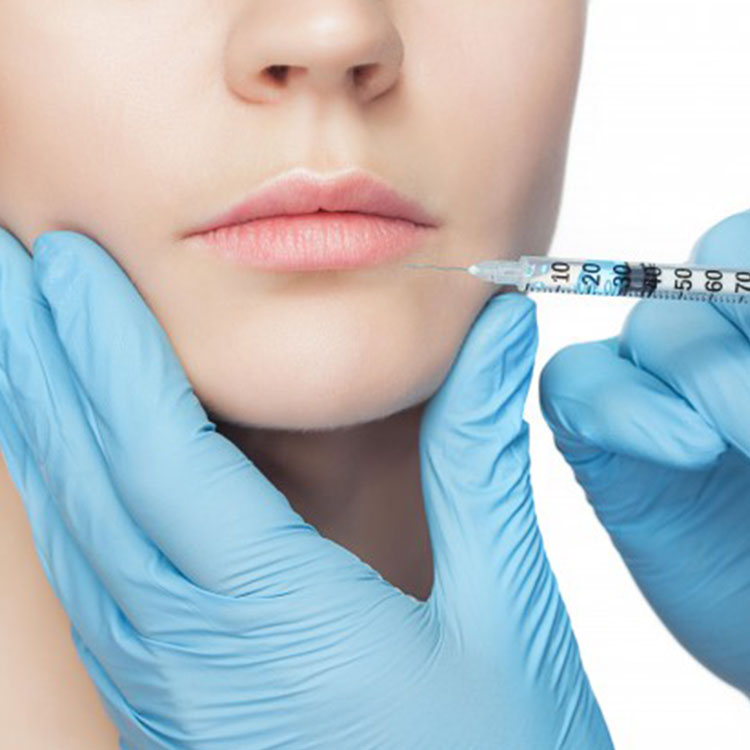
Sinae Acidum hyaluronic Iniectio Manufacturers, Suppliers, Factory
AMHWA® fabricatores et praebitores in Sinis professio est. Qualitas nostra princeps Acidum hyaluronic Iniectio non solum est tardus venditionis ac vilis pretiis praebens. Gratam officinam nostram emere emendas novissimas fructus et officia.
Hot Products
Sodium Hyaluronate Food Grade
AMHWA® ut opificem professionalem, volumus tibi dare qualitatem altam Sodium Hyaluronate Victus Gradus. Et offeremus tibi optimam post venditionem servitii et opportunam traditionem.Hydrolyzed Sodium Hyaluronate Food Grade
AMHWA® ut professionalis altitudinis qualitas Hydrolyzed Sodium Hyaluronate Food Grade opificem, certificare potes ut Gradus Cibus Hydrolyzed Sodium Hyaluronatum ex officina nostra emeret et tibi optimam post venditionem servitii et partus opportune offeremus.Crux-coniunctum Hyaluronic Acidum Lidocaine
AMHWA® una est e cruce-coniuncto acido hyaluronico cum Lidocain fabrica et praebitoribus in Sinis. AMHWA BIOPHARM, Fabrica mundi primi acidi hyaluronici. Summus qualitas products multis agris ut medicina, cutis cura, et operando cibum circa mundum.Hydrolyzed Hyaluronic Acidum
Gratus es venire ad officinam nostram ut emendum novissimam venditionem, humilem, et qualitatem hyaluronicum acidum hydrolyticum, AMHWA® cooperandum tibi prospiciat.Sodium hyaluronate medicamine gradu
Sodium Hyaluronate Cosmetic Grade, is a highly hydrophilic molecule, plays an important role in tissue hydrodynamics and contributes to the transport of water, it helps to maintain the hydration and elastoviscosity of tissues.The remarkable viscoelastic and water holding property of HA, besides its biocompatibility, biodegradability, and non-immunogenicity, has increased its appeal in numerous medical and cosmetic applications."Sodium hyaluronate I% solution
Hyaluronic Acid (Sodium Hyaluronate 1% Solution), widely exists in the extracellular matrix of animal tissues and the capsule of Streptococcus. HA belongs to mucopolysaccharide and is linear un-branched, high molecular weight polysaccharides containing a repeating disaccharide unit (D-glucuronic acid and N-acetyl glucosamine). HA have some unique properties and functions, such as water retention, lubrication, viscosity and good biocompatibility, so HA is widely applied to cosmetics, health food, Beauty filling operation and pharmaceutics,etc.